Battery Information
- Alkaline & Zinc Carbon Battery Information
- Button Cell Information
- Lithium Battery Information
- Lithium Ion Battery Information
- Nickel Cadmium Battery Cell Information
- Silver Zinc Batteries

Alkaline & Zinc Carbon Battery Information |
\"Heavy duty\" is a meaningless marketing term. Those batteries are good old carbon-zinc. Just like the ones that leaked and ruined all you cool toys in the 50s. \"Heavy-duty\" was invented back then to imply a better grade of carbon-zinc batteries.
For almost all applications, alkaline are better than carbon-zinc.
\"Heavy duty\" and alkaline batteries only have slightly different chemistries, and slightly different open circuit voltages. Both are very close to the established \'label voltage\' of 1.5v for the carbon-zinc wet cell (Leclanché, 1868) and later the carbon-zinc dry cell (Gassner, 1888). By comparison, the cell voltage of a lead-acid battery is ~2.0v, and NiCad cells run about 1.4v
However, there are significant differences in practice - mostly length of life (Alkaline last 4-9 times as long), current discharge properties (alkaline supply more current for longer), voltage drop-off with use (\"Heavy duty\" drop off gradually as they are drained; alkaline keep a higher voltage until they are nearer their end of life, and then drop off) etc. Sometimes a device requires a certain voltage to operate, and the gradual voltage drop-off of a \"heavy duty\" cell causes it to fail even when it has significant charge left in it. Therefore, a heavy duty battery that \'died\' in 10 minutes in one device, may still work fine in others
The term \"heavy duty\" is a misnomer. It was \"heavy duty\" in the 60s when I was born (and probably well before) but only in comparison to the older cells. Today, it should be called \"pathetic underachiever\" but somehow the makers are slow to make the change.
The \"regular duty\" dry cell was basically a Leclanche cell that mixed the electrolyte into a slurry with black manganese dioxide instead of using a plain liquid. The electrolyte was a slightly acidic mixture of ammonium chloride [and some zinc chloride], and actually had an \'open circuit voltage\' of 1.55v, that settled close to 1.5v under load.
The anode (- end) is actually the zinc casing itself (hidden under steel end plates for durability and a protective paper shell) The cathode (+ end) is a carbon bar, inserted into the electrolyte, and sealed with insulating wax, to make both sides of the cell accessible from the outside, without shorting out the cell.. Some of the zinc in the casing is consumed during use, so a heavily discharged dry cell may \'break through\' and leak if left in the circuit.
(The terms cathode and anode may seem reversed here. That\'s because battery designers use + and - relative to the cell chemistry, not the voltage supplied by the battery - or so I\'ve read. It still seems silly to me.)
The \'heavy duty\' battery changed the primary electrolyte to zinc chloride resulting in a slightly higher open cell voltage (~1.6v), and held its voltage better under load, as well as having a longer life (more pronounced under heavier loads than light loads)
Despite the way the term is used in advertising, alkaline cells are actually chemically carbon-zinc cells with a somewhat improved [predictably, more alkaline] electrolyte. They have a slightly higher open cell voltage, more storage capacity, and less voltage drop during the main part of the discharge curve, but most of its benefits actually come from improved construction.
The casing of an alkaline cell is just protective. The Anode is a gel of zinc and KOH (a potent alkali) inside a polyester cylinder in the middle of the battery. The gel is connected to the external - terminal by a brass spike. The cathode is a shell of carbon+manganese dioxide slurry between the polyester cylinder and the outer casing
Alkaline cells can last 4-9 times as long as \"heavy duty\" cells (depending on the application) and have a better discharge curve (they lose less voltage as they are discharged, then drop of more at end of life]. They can\'t be recharged (they tend to explode) while the heavy duty cells could be mostly recharged a few times. Standard and heavy duty rechargers were common in the 1970s, though recharging was always recommended against by the manufacturers, because of the risk of leakage/bursting after erosion of the zinc casing, and lost profit from erosion in new battery sales.
However, the Renewal cell (Rayovac) is actually a carbon-zinc cell that is designed to be recharged 25-100 times (using a microprocessor-based recharger). A NiCad battery can be recharged 1000 times ideally but its chemistry has a lower open cell voltage of about 1.4v, which is fine for many applications, but won\'t work in others. (Carbon zinc cells don\'t reach 1.4 until they are mostly dead) Since few people recharged NiCad’s that often except in specialty uses like laptops, and NiCad’s had other issues, the Renewal was considered a viable alternative to NiCad.
NiMH [Nickel Metal hydride] rechargeable cells have more capacity than NiMH but have an open cell voltage of ca. 1.2 v - not suitable for many applications. For this reason many devices specify alkaline cells (heavy duty should work, due to the similar open cell voltage, but they\'ll have a much shorter life
Motorcycle, car and UPS batteries use 3 or 6 lead acid cells (2v each) in series to achieve their rated voltage of 6v or 12v; the rectangular 9v \"transistor\" battery contains six 1.5v batteries in series inside it. You may have never seen a \"B\" cell, because in the US, it was a stacked series of cells that added up to 45-90v, and was used for vacuum tubes. It\'s called a B cell because \"B voltage\" was an existing term in vacuum tubes. In Asia, however, there was a B cell that was about as long as a D cell, but narrower than a C cell. Kind of like a supersized A cell. I have one, but it\'s been dead for 20 years.

Button Cell Information |
Button, coin, or watch cells
A watch battery or button cell is a small single cell battery shaped as a squat cylinder typically 5 to 12 mm in diameter and 1 to 6 mm high—like a button on a garment, hence the name. Button cells are used to power small portable electronics devices such as wrist watches, pocket calculators, and hearing aids. Some cells larger than the dimensions above are also called button cells, but are less commonly used. Lithium cells are generally similar but somewhat larger; they tend to be called either lithium cells or batteries or coin cells rather than button cells.
Devices using button cells are usually designed to use a cell giving a long service life, typically well over a year in continuous use in a wristwatch. Most button cells have low self-discharge and hold their charge for a long time if not used. Higher-power devices such as hearing aids, where high capacity is important and low self-discharge less so as the cell will usually be used up before it has time to discharge, may use zinc-air cells which have much higher capacity for a given size, but discharge over a few weeks even if not used.
Button cells are single cells, usually disposable primary cells. Common anode materials are zinc or lithium. Common cathode materials are manganese dioxide, silver oxide, carbon monofluoride, cupric oxide or oxygen from the air. Mercuric oxide button cells were formerly common, but are no longer available due to the toxicity and environmental hazard of mercury.
Cells have a metal can forming the bottom body, with a circular insulated top cap. The can is the positive and the top the negative terminal.
Cells of different chemical composition made in the same size are mechanically interchangeable. However, the composition can affect service life and voltage stability. Using the wrong cell may lead to short life or improper operation (for example, light metering on a camera requires a stable voltage, and silver cells are usually specified). Sometimes different cells of the same type and size and specified capacity in mAh are optimised for different loads by using different electrolytes, so that one may have longer service life, than the other if supplying a relatively high current.
Properties of different types
Silver cells may have very stable output voltage until it suddenly drops very rapidly at end of life. This varies for individual types; one manufacturer (Energizer) offers 3 silver oxide cells of the same size, 357-303, 357-303H,and EPX76, with capacities ranging from 150 to 200 mAh, voltage characteristics ranging from gradually reducing to fairly constant, and some stated to be for continuous low drain with high pulse on demand, others for photo use.
Mercury batteries also supply a stable voltage, but are now banned in many countries due to their toxicity and environmental impact.
Alkaline batteries are made in the same button sizes as other types, but typically provide less capacity and less stable voltage (it drops gradually in use) than more costly silver oxide or lithium cells. They are often sold as cheap watch batteries to, and sometimes by, people who do not know the difference.[1]
Zinc-air batteries use air as the depolarizer and have much higher capacity than other types (they use air from the atmosphere which does not need to be supplied in the battery). A seal is removed before use to allow air to enter the cell; the cell will then self-discharge in a few weeks even if not used up.
For comparison, a cell of diameter 11.6 mm and height 5.4 mm from one reputable manufacturer has the following properties.[2] In many cases there are several batteries of the same chemistry and size with different capacities and properties; figures listed are merely indicative.
- Silver: capacity 200 mAh to an end-point of 0.9 V, internal resistance 5–15 ohms, weight 2.3 g
- Alkaline (manganese dioxide): 150 mAh (0.9), 3-9 ohms, 2.4 g
- Mercury 200mAh, 2.6 g
- Zinc-air 620 mAh, 1.9 g
Examining datasheets for a manufacturer's range[2] may find a high-capacity alkaline cell with a capacity as high as one of the lower-capacity silver types; or a particular silver cell with twice the capacity of some particular alkaline cell. If the powered equipment requiring a relatively high voltage (e.g., 1.3V) to operate correctly, a silver cell with a flat discharge characteristic will give much longer service than an alkaline cell—even if it has the same specified capacity in mAh to an end-point of 0.9V. If some device seems to "eat up" batteries after the original supplied by the manufacturer is replaced, it may be useful to check the device's requirements and the replacement battery's characteristics. For digital calipers, in particular, some are specified to require at least 1.25V to operate, others 1.38V.[3][4]
Datasheets for some cheaper cells, particularly alkaline, are not available, so it is not possible to say whether capacities are about the same as for documented types.[5] Discussions on web forums suggest that they can be very poor.[6]
In some ways the size is the most important property of a button cell: cells of different chemistry are to a considerable extent interchangeable. In practice only cells of fairly similar voltages are made in any given size; there is no "CR1154" 3V lithium battery mechanically interchangeable with a 1.5V silver or alkaline size 1154 cell. Use of a battery of significantly higher voltage than equipment is designed for can cause permanent damage, but use of a cell of the right voltage but unsuitable characteristics can only lead to short battery life or failure to operate equipment.
Electrochemical system
The first letter identifies the chemical composition of the battery, which also implies a nominal voltage:
|
Letter |
Common |
Positive |
Electrolyte |
Negative |
Nominal |
End-point |
|
L |
Alkaline |
Manganese dioxide |
Alkali |
Zinc |
1.5 |
1.0 |
|
S |
Silver |
Silver oxide |
Alkali |
Zinc |
1.55 |
1.2 |
|
P |
Zinc-air |
Oxygen |
Alkali |
Zinc |
1.4 |
1.2 |
|
C |
Lithium |
Manganese dioxide |
Organic |
Lithium |
3 |
2.0 |
|
B |
Carbon monofluoride |
Organic |
Lithium |
3 |
2.0 |
|
|
G |
Copper oxide |
Organic |
Lithium |
1.5 |
1.2 |
|
|
M,N(withdrawn) |
Mercury |
Mercuric oxide |
Alkaline |
Zinc |
1.35/1.40 |
1.1 |

Lithium Battery Information |
CR2032 lithium button cell battery
Lithium 9 volt, AA, &AAA sizes
Lithium batteries are disposable (primary) batteries that have lithium metal or lithium compounds as an anode. Depending on the design and chemical compounds used, lithium cells can produce voltages from 1.5 V to about 3.7 V, over twice the voltage of an ordinary zinc–carbon battery or alkaline battery.[1] Lithium batteries are widely used in products such as portable consumer electronic devices.
Lithium primary batteries account for 28% of all primary battery sales in Japan but only 1% of all battery sales in Switzerland. In the UK and EU only 0.5% of all battery sales including secondary types are lithium primaries.
Description
The term "lithium battery" refers to a family of different chemistries, comprising many types of cathodes and electrolytes.
The most common type of lithium cell used in consumer applications uses metallic lithium as anode and manganese dioxide as cathode, with a salt of lithium dissolved in an organic solvent.
Disassembled CR2032 battery From left — negative cup from inner side with layer of lithium (oxidized in air), separator(porous material), cathode (manganese dioxide), metal grid — current collector, metal casing (+)(damaged during opening the cell), on the bottom is plastic sealing ring
Another type of lithium cell having a large energy density is the lithium-thionyl chloride cell. Lithium-thionyl chloride batteries are generally not sold to the consumer market, and find more use in commercial/industrial applications, or are installed into devices where no consumer replacement is performed. In this cell, a liquid mixture of thionyl chloride(SOCl2) and lithium tetrachloroaluminate (LiAlCl4) acts as the electrolyte and cathode respectively. A porous carbon material serves as a cathode current collector which receives electrons from the external circuit. Lithium-thionyl chloride batteries are well suited to extremely low-current applications where long life is necessary, such as wireless alarm systems.
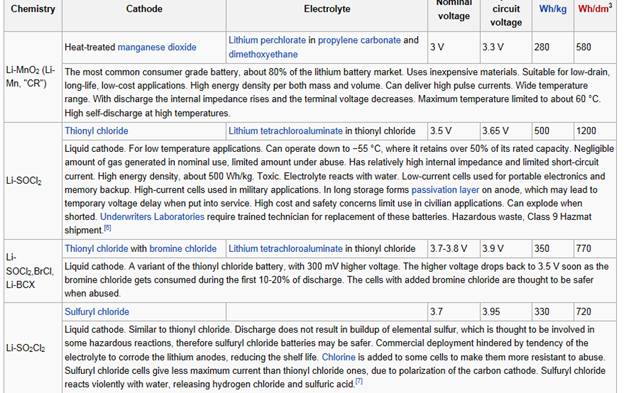

Lithium Ion Battery Information |
A lithium-ion battery (sometimes Li-ion battery or LIB) is a family of rechargeable battery types in which lithium ions move from the negative electrode to the positive electrode during discharge, and back when charging. Chemistry, performance, cost, and safety characteristics vary across LIB types. Unlike lithium primary batteries (which are disposable), lithium-ion electrochemical cells use an intercalated lithium compound as the electrode material instead of metallic lithium.
Lithium-ion batteries are common in consumer electronics. They are one of the most popular types of rechargeable battery for portable electronics, with one of the best energy densities, no memory effect, and only a slow loss of charge when not in use. Beyond consumer electronics, LIBs are also growing in popularity for military, electric vehicle, and aerospace applications.[6] Research is yielding a stream of improvements to traditional LIB technology, focusing on energy density, durability, cost, and intrinsic safety.
The three primary functional components of a lithium-ion battery are the negative electrode, positive electrode, and the electrolyte. The negative electrode of a conventional lithium-ion cell is made from carbon. The positive electrode is a metal oxide, and the electrolyte is a lithium salt in an organic solvent.[8] The electrochemical roles of the electrodes change between anode and cathode, depending on the direction of current flow through the cell.
The most commercially popular negative electrode material is graphite. The positive electrode is generally one of three materials: a layered oxide (such as lithium cobalt oxide), a polyanion (such as lithium iron phosphate), or a spinel (such as lithium manganese oxide).[9]
The electrolyte is typically a mixture of organic carbonates such as ethylene carbonate or diethyl carbonate containing complexes of lithium ions.[10] These non-aqueous electrolytes generally use non-coordinating anion salts such as lithium hexafluorophosphate (LiPF6), lithium hexafluoroarsenate monohydrate (LiAsF6), lithium perchlorate (LiClO4), lithium tetrafluoroborate (LiBF4), and lithium triflate (LiCF3SO3).
Depending on materials choices, the voltage, capacity, life, and safety of a lithium-ion battery can change dramatically. Recently, novel architectures using nanotechnology have been employed to improve performance.
Pure lithium is very reactive. It reacts vigorously with water to form lithium hydroxide and hydrogen gas. Thus, a non-aqueous electrolyte is typically used, and a sealed container rigidly excludes water from the battery pack.
Lithium ion batteries are more expensive than NiCd batteries but operate over a wider temperature range with higher energy densities, while being smaller and lighter. They are fragile and so need a protective circuit to limit peak voltages.
Li-ion cells are available in various formats, which can generally be divided into four groups:[11][12]
- Small cylindrical (solid body without terminals, such as those used in laptop batteries)
- Large cylindrical (solid body with large threaded terminals)
- Pouch (soft, flat body, such as those used in cell phones)
- Prismatic (semi-hard plastic case with large threaded terminals, often used in vehicles' traction packs)
The lack of case gives pouch cells the highest energy density; however, pouch cells (and prismatic cells) require an external means of containment to prevent expansion when their state-of-charge (SOC) level is high.[13]
| Positive electrodes | |||
|
Electrode material |
Average potential difference |
Specific capacity |
Specific energy |
|
LiCoO2 |
3.7 V |
140 mA·h/g |
0.518 kW·h/kg |
|
LiMn2O4 |
4.0 V |
100 mA·h/g |
0.400 kW·h/kg |
|
LiNiO2 |
3.5 V |
180 mA·h/g |
0.630 kW·h/kg |
|
LiFePO4 |
3.3 V |
150 mA·h/g |
0.495 kW·h/kg |
|
Li2FePO4F |
3.6 V |
115 mA·h/g |
0.414 kW·h/kg |
|
LiCo1/3Ni1/3Mn1/3O2 |
3.6 V |
160 mA·h/g |
0.576 kW·h/kg |
|
Li(LiaNixMnyCoz)O2 |
4.2 V |
220 mA·h/g |
0.920 kW·h/kg |
|
Negative electrodes |
|||
|
Electrode material |
Average potential difference |
Specific capacity |
Specific energy |
|
Graphite (LiC6) |
0.1-0.2 V |
372 mA·h/g |
0.0372-0.0744 kW·h/kg |
|
Hard Carbon (LiC6) |
? V |
450 mA·h/g |
? kW·h/kg |
|
Titanate (Li4Ti5O12) |
1-2 V |
160 mA·h/g |
0.16-0.32 kW·h/kg |
|
Si (Li4.4Si)[38] |
0.5-1 V |
4212 mA·h/g |
2.106-4.212 kW·h/kg |
|
Ge (Li4.4Ge)[39] |
0.7-1.2 V |
1624 mA·h/g |
1.137-1.949 kW·h/kg |
Advantages
A lithium-ion battery from a laptop computer
- Wide variety of shapes and sizes efficiently fitting the devices they power.
- Much lighter than other energy-equivalent secondary batteries.[45]
- High open circuit voltage in comparison to aqueous batteries (such as lead acid, nickel-metal hydride and nickel-cadmium).[46] This is beneficial because it increases the amount of power that can be transferred at a lower current.
- No memory effect.
- Self-discharge rate of approximately 5-10% per month, compared to over 30% per month in common nickel metal hydride batteries, approximately 1.25% per month for Low Self-Discharge NiMH batteries and 10% per month in nickel-cadmium batteries.[47] According to one manufacturer, lithium-ion cells (and, accordingly, "dumb" lithium-ion batteries) do not have any self-discharge in the usual meaning of this word.[35] What looks like a self-discharge in these batteries is a permanent loss of capacity (see Disadvantages). On the other hand, "smart" lithium-ion batteries do self-discharge, due to the drain of the built-in voltage monitoring circuit.
- Components are environmentally safe as there is no free lithium metal.[citation needed]
Disadvantages
Cell life
- Charging forms deposits inside the electrolyte that inhibit ion transport. Over time, the cell's capacity diminishes. The increase in internal resistance reduces the cell's ability to deliver current. This problem is more pronounced in high-current applications. The decrease means that older batteries do not charge as much as new ones (charging time required decreases proportionally).
- High charge levels and elevated temperatures (whether from charging or ambient air) hasten capacity loss.[48] Charging heat is caused by the carbon anode (typically replaced with lithium titanate which drastically reduces damage from charging, including expansion and other factors).[49]
Internal resistance
- The internal resistance of standard (Cobalt) lithium-ion batteries is high compared to both other rechargeable chemistries such as nickel-metal hydride and nickel-cadmium, and LiFePO4 and lithium-polymer cells.[50] Internal resistance increases with both cycling and age.[51][52][53] Rising internal resistance causes the voltage at the terminals to drop under load, which reduces the maximum current draw. Eventually increasing resistance means that the battery can no longer operate for an adequate period.
- To power larger devices, such as electric cars, connecting many small batteries in a parallel circuit is more effective[54] and efficient than connecting a single large battery.[55]
Safety requirements
If overheated or overcharged, Li-ion batteries may suffer thermal runaway and cell rupture.[56] In extreme cases this can lead to combustion. Deep discharge may short-circuit the cell, in which case recharging would be unsafe.[57] To reduce these risks, Lithium-ion battery packs contain fail-safe circuitry that shuts down the battery when its voltage is outside the safe range of 3–4.2 V per cell. When stored for long periods the small current draw of the protection circuitry itself may drain the battery below its shut down voltage; normal chargers are then ineffective. Many types of lithium-ion cell cannot be charged safely below 0°C.[58]
Other safety features are required in each cell:
- Shut-down separator (for overtemperature)
- Tear-away tab (for internal pressure)
- Vent (pressure relief)
- Thermal interrupt (overcurrent/overcharging)
These devices occupy useful space inside the cells, add additional points of failure and irreversibly disable the cell when activated. They are required because the anode produces heat during use, while the cathode may produce oxygen. These devices and improved electrode designs reduce/eliminate the risk of fire or explosion.
These safety features increase costs compared to nickel metal hydride batteries, which require only a hydrogen/oxygen recombination device (preventing damage due to mild overcharging) and a back-up pressure valve.[47]
Lithium-thionyl chloride (Li-SOCl2)
Lithium-thionyl chloride cells have a metallic lithium anode (the lightest of all the metals) and a liquid cathode comprising a porous carbon current collector filled with thionyl chloride (SOCl2). They deliver a voltage of 3.6 V and are cylindrical in shape, in 1/2AA to D format, with spiral electrodes for power applications and bobbin construction for prolonged discharge.
Lithium-thionyl chloride cells have a high energy density, partly because of their high nominal voltage of 3.6 V. Bobbin versions can reach 1220 Wh/L and 760 Wh/kg, for a capacity of 18.5 Ah at 3.6 V in D format. Because self-discharge is extremely low (less than 1% per year), this kind of cell can support long storage periods and achieve a service life of 10 to 20 years.
MAJOR APPLICATION |
? Digital lithium batteries
? TPMS
? Computer main-board
? Memory card
? Calculator
? Electronic clock & watch
? Thief alarm
? Electronic dictionary
? Water meters
? Calorimeters
? Apparatus and instrument
? Various types of military electronics or communication equipments
| MAJOR FEATURES |
? High energy density
? Long shelf life
? Wide operating temperature
? Good sealing feature
? Steady discharge voltage
Lithium Ion Polymer Battery
Lithium-ion polymer batteries, polymer lithium ion, or more commonly lithium polymer batteries (abbreviated Li-poly, Li-Pol, LiPo, LIP, PLI or LiP) are rechargeable (secondary cell) batteries. LiPo batteries are usually composed of several identical secondary cells in parallel to increase the discharge current capability.
The advantages of Li-ion polymer over the lithium-ion design include potentially lower cost of manufacture, adaptability to a wide variety of packaging shapes, reliability, and ruggedness. Lithium-ion polymer batteries started appearing in consumer electronics around 1995.
Cells sold today as polymer batteries are pouch cells. Unlike lithium-ion cylindrical cells, which have a rigid metal case, pouch cells have a flexible, foil-type (polymer laminate) case. In cylindrical cells, the rigid case presses the electrodes and the separator onto each other; whereas in polymer cells this external pressure is not required (or often used) because the electrode sheets and the separator sheets are laminated onto each other. Since individual pouch cells have no strong metal casing, by themselves they are over 20% lighter than equivalent cylindrical cells.
A compelling advantage of Li-poly cells is that manufacturers can shape the battery almost however they please, which can be important to mobile phone manufacturers constantly working on smaller, thinner, and lighter phones.
Li-poly batteries are also gaining favor in the world of radio-controlled aircraft as well as radio-controlled cars, where the advantages of both lower weight and greatly increased run times and power delivery can be sufficient justification for the price. Radio-controlled car batteries are often protected by durable plastic cases to prevent puncture. Specially designed electronic motor speed controls are used to prevent excessive discharge and subsequent battery damage. This is achieved using a low voltage cutoff (LVC) setting that is adjusted to maintain cell voltage greater than (typically) 3 V per cell.
Li-poly batteries are also gaining ground in PDAs and laptop computers, such as Apple's MacBook family, Amazon's Kindle, Lenovo's Thinkpad X300 and Ultrabay Batteries, the OQO series of palmtops, the HP Mini and Dell products featuring D-bay batteries. Very small GPS tracking units such as Garmin's GTU-10 rely on li-poly batteries for days or even weeks of autonomous operation between recharges. Li-poly batteries are also used in small digital music devices such as iPods, Zunes, and other MP3 players and the Apple iPhone and iPad, as well as gaming equipment like Sony's PlayStation 3 wireless controllers. They are desirable in applications where small form factors and energy density outweigh cost considerations.
These batteries may also power the next generation of battery electric vehicles. The cost of an electric car of this type is currently significantly higher than of a petrol car, but it is likely that with increased production and technological advances, the cost of Li-poly batteries will go down.
Hyundai Motor Company uses this battery type in some of its hybrid electric vehicles. On 26 October 2010, a Li-poly powered Audi A2 covered the record distance of 600 km without recharging. From April 2011 batteries of this type for output exceeding one Megawatt have been responsible for a number of world speed records in drag racing.
- All Li-Ion cells expand at high levels of state of charge (SOC); if uncontained, this may result in delamination, and reduction of reliability and cycle life; the case of cylindrical cells provides that containment, while pouch cells, by themselves, are not contained. Therefore, to achieve the rated performance, a battery composed of pouch cells must include a strong external casing to retain its shape.
- Overcharging a Li-poly battery can cause an explosion or fire.
- During discharge on load, the load has to be removed as soon as the voltage drops below approximately 3.0 Vper cell (used in a series combination), or else the batterywill subsequently no longer accept a full charge and may experience problems holding voltage under load. Li-poly batteries can be protected by circuitry that prevents over-charge and deep-discharge.
- Li-poly batteries typically require more than an hour for a full charge.
- Compared to the lithium-ion battery, Li-poly has a greater life cycle degradation rate.
- Lithium polymer-specific chargers are required in order to avoid fire and explosion.
- Explosions can also occur if the battery is short-circuited, as tremendous current passes through the cell in an instant. Radio-control enthusiasts take special precautions to ensure their battery leads are properly connected and insulated. Furthermore fires can occur if the cell or pack is punctured.
- As these lithium polymer batteries first emerged in the market, they were expensive.
- While charging the lithium polymer batteries, the individual cells in the pack should be charged evenly. For this purpose, the cells are to be charged with special chargers. This entails special care while charging the batteries in addition to incurring expenses on procuring the chargers specific to lithium polymer batteries.
Lithium-Ion Phosphate Battery
The lithium iron phosphate (LiFePO4) battery, also called LFP battery, is a type of rechargeable battery, specifically a lithium-ion battery, which uses LiFePO4 as a cathode material.
The LiFePO4 battery uses a lithium-ion-derived chemistry and shares many advantages and disadvantages with other Lithium-ion battery chemistries. However, there are significant differences.
LFP chemistry offers a longer cycle life than other lithium-ion approaches.[6]
The use of phosphates avoids cobalt's cost and environmental concerns, particularly concerns about cobalt entering the environment through improper disposal.[6]
LiFePO4 has higher current or peak-power ratings than LiCoO2.[7]
The energy density (energy/volume) of a new LFP battery is some 14% lower than that of a new LiCoO2 battery.[8] Also, many brands of LFPs have a lower discharge rate than lead-acid or LiCoO2. Since discharge rate is a percentage of battery capacity a higher rate ca

Nickel Cadmium Battery Cell Information |
The nickel–cadmium battery(Ni–Cd battery) (commonly abbreviated NiCd or NiCad) is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes.
The abbreviation NiCad is a registered trademark of SAFT Corporation, although this brand name is commonly used to describe all Ni–Cd batteries. The abbreviation NiCd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd).
There are two types of Ni–Cd batteries: sealed and vented. This article mainly deals with sealed cells.
Applications:
Sealed Ni–Cd cells may be used individually, or assembled into battery packs containing two or more cells. Small cells are used for portable electronics and toys, often using cells manufactured in the same sizes as primary cells. When Ni–Cd batteries are substituted for primary cells, the lower terminal voltage and smaller ampere-hour capacity may reduce performance as compared to primary cells. Miniature button cells are sometimes used in photographic equipment, hand-held lamps (flashlight or torch), computer-memory standby, toys, and novelties.
Specialty Ni–Cd batteries are used in cordless and wireless telephones, emergency lighting, and other applications. With a relatively low internal resistance, they can supply high surge currents. This makes them a favourable choice for remote-controlled electric model airplanes, boats, and cars, as well as cordless power tools and camera flash units. Larger flooded cells are used for aircraft starting batteries, electric vehicles, and standby power.
Ni–Cd cells have a nominal cell potential of 1.2 volts (V). This is lower than the 1.5 V of alkaline and zinc–carbon primary cells, and consequently they are not appropriate as a replacement in all applications. However, the 1.5 V of a primary alkaline cell refers to its initial, rather than average, voltage. Unlike alkaline and zinc–carbon primary cells, a Ni–Cd cell's terminal voltage only changes a little as it discharges. Because many electronic devices are designed to work with primary cells that may discharge to as low as 0.90 to 1.0 V per cell, the relatively steady 1.2 V of a Ni–Cd cell is enough to allow operation. Some would consider the near-constant voltage a drawback as it makes it difficult to detect when the battery charge is low.
Ni–Cd batteries used to replace 9 V batteries usually only have six cells, for a terminal voltage of 7.2 volts. While most pocket radios will operate satisfactorily at this voltage, some manufacturers such as Varta made 8.4 volt batteries with seven cells for more critical applications.
12 V Ni–Cd batteries are made up of 10 cells connected in series.
Battery characteristics
Comparison with other batteries:
Recently, nickel–metal hydride and lithium-ion batteries have become commercially available and cheaper, the former type now rivaling Ni–Cd batteries in cost. Where energy density is important, Ni–Cd batteries are now at a disadvantage compared with nickel–metal hydride and lithium-ion batteries. However, the Ni–Cd battery is still very useful in applications requiring very high discharge rates because it can endure such discharge with no damage or loss of capacity.
Advantages:
When compared to other forms of rechargeable battery, the Ni–Cd battery has a number of distinct advantages:
- The batteries are more difficult to damage than other batteries, tolerating deep discharge for long periods. In fact, Ni–Cd batteries in long-term storage are typically stored fully discharged. This is in contrast, for example, to lithium ion batteries, which are less stable and will be permanently damaged if discharged below a minimum voltage.
- Ni–Cd batteries typically last longer, in terms of number of charge/discharge cycles, than other rechargeable batteries such as lead/acid batteries.
- Compared to lead–acid batteries, Ni–Cd batteries have a much higher energy density. A Ni–Cd battery is smaller and lighter than a comparable lead–acid battery. In cases where size and weight are important considerations (for example, aircraft), Ni–Cd batteries are preferred over the cheaper lead–acid batteries.
- In consumer applications, Ni–Cd batteries compete directly with alkaline batteries. A Ni–Cd cell has a lower capacity than that of an equivalent alkaline cell, and costs more. However, since the alkaline battery's chemical reaction is not reversible, a reusable Ni–Cd battery has a significantly longer total lifetime. There have been attempts to create rechargeable alkaline batteries, or specialized battery chargers for charging single-use alkaline batteries, but none that has seen wide usage.
- The terminal voltage of a Ni–Cd battery declines more slowly as it is discharged, compared with carbon–zinc batteries. Since an alkaline battery's voltage drops significantly as the charge drops, most consumer applications are well equipped to deal with the slightly lower Ni–Cd cell voltage with no noticeable loss of performance.
- The capacity of a Ni–Cd battery is not significantly affected by very high discharge currents. Even with discharge rates as high as 50C, a Ni–Cd battery will provide very nearly its rated capacity. By contrast, a lead acid battery will only provide approximately half its rated capacity when discharged at a relatively modest 1.5C.
- Nickel–metal hydride (NiMH) batteries are the newest, and most similar, competitor to Ni–Cd batteries. Compared to Ni–Cd batteries, NiMH batteries have a higher capacity and are less toxic, and are now more cost effective. However, a Ni–Cd battery has a lower self-discharge rate (for example, 20% per month for a Ni–Cd battery, versus 30% per month for a traditional NiMH under identical conditions), although low self-discharge NiMH batteries are now available, which have substantially lower self-discharge than either Ni–Cd or traditional NiMH batteries. This results in a preference for Ni–Cd over NiMH batteries in applications where the current draw on the battery is lower than the battery's own self-discharge rate (for example, television remote controls). In both types of cell, the self-discharge rate is highest for a full charge state and drops off somewhat for lower charge states. Finally, a similarly sized Ni–Cd battery has a slightly lower internal resistance, and thus can achieve a higher maximum discharge rate (which can be important for applications such as power tools).
Disadvantages:
- The primary trade-off with Ni–Cd batteries is their higher cost and the use of cadmium. This heavy metal is an environmental hazard, and is highly toxic to all higher forms of life. They are also more costly than lead–acid batteries because nickel and cadmium cost more.
- One of the biggest disadvantages is that the battery exhibits a very marked negative temperature coefficient. This means that as the cell temperature rises, the internal resistance falls. This can pose considerable charging problems, particularly with the relatively simple charging systems employed for lead–acid type batteries. Whilst lead–acid batteries can be charged by simply connecting a dynamo to them, with a simple electromagnetic cut-out system for when the dynamo is stationary or an over-current occurs, the Ni–Cd battery under a similar charging scheme would exhibit thermal runaway, where the charging current would continue to rise until the over-current cut-out operated or the battery destroyed itself. This is the principal factor that prevents its use as engine-starting batteries. Today with alternator-based charging systems with solid-state regulators, the construction of a suitable charging system would be relatively simple, but the car manufacturers are reluctant to abandon tried-and-tested technology.
Availability:
Ni–Cd cells are available in the same sizes as alkaline batteries, from AAA through D, as well as several multi-cell sizes, including the equivalent of a 9 volt battery. A fully charged single Ni–Cd cell, under no load, carries a potential difference of between 1.25 and 1.35 volts, which stays relatively constant as the battery is discharged. Since an alkaline battery near fully discharged may see its voltage drop to as low as 0.9 volts, Ni–Cd cells and alkaline cells are typically interchangeable for most applications.
In addition to single cells, batteries exist that contain up to 300 cells (nominally 360 volts, actual voltage under no load between 380 and 420 volts). This many cells are mostly used in automotive and heavy-duty industrial applications. For portable applications, the number of cells is normally below 18 cells (24V). Industrial-sized flooded batteries are available with capacities ranging from 12.5Ah up to several hundred Ah.
Nickel-metal hydride battery:
A nickel–metal hydride cell, abbreviated NiMH or Ni-MH, is a type of rechargeable battery. It is very similar to the nickel–cadmium cell (NiCd). NiMH use positive electrodes of nickel oxyhydroxide (NiOOH), like the NiCd, but the negative electrodes use a hydrogen-absorbing alloy instead of cadmium. A NiMH battery can have two to three times the capacity of an equivalent size NiCd, and their energy density approaches that of a lithium-ion cell.
The typical specific energy for small NiMH cells is about 100 W·h/kg, and for larger NiMH cells about 75 W·h/kg (270 kJ/kg). This is significantly better than the typical 40–60 W·h/kg for Ni–Cd, and similar to the 100-160 W·h/kg for Li-ion. NiMH has a volumetric energy density of about 300 W·h/L (1080 MJ/m³), significantly better than nickel–cadmium at 50–150 W·h/L, and about the same as li-ion at 250-360 W·h/L.
NiMH batteries have replaced NiCd for many roles, notably small rechargeable batteries. NiMH batteries are very common for AA (penlight-size) batteries, which have nominal charge capacities (C) ranging from 1100 mA·h to 3100 mA·h at 1.2 V, measured at the rate that discharges the cell in five hours. Useful discharge capacity is a decreasing function of the discharge rate, but up to a rate of around 1×C (full discharge in one hour), it does not differ significantly from the nominal capacity.[4] NiMH batteries normally operate at 1.2 V per cell, somewhat lower than conventional 1.5 V cells, but will operate most devices designed for that voltage.
About 22% of portable rechargeable batteries sold in Japan in 2010 were NiMH. In Switzerland in 2009, the equivalent statistic was approximately 60%. This percentage has fallen over time due to the increase in manufacture of li-ion batteries: in 2000, almost half of all portable rechargeable batteries sold in Japan were NiMH.
One significant disadvantage of NiMH batteries is a high rate of self-discharge; a NiMH battery will lose as much as 3% of its charge per week of storage. In 2005 a low self-discharge NiMH battery (LSD) was developed. LSD NiMH batteries significantly lower self-discharge, but at the cost of lowering capacity by about 20%.
History
The first consumer grade NiMH cells for smaller applications appeared on the market in 1989, the culmination of over two decades of research and development
The earliest pioneering work on NiMH batteries — essentially based on sintered Ti2Ni+TiNi+x alloys for the negative electrode and NiOOH-electrodes for the positives — was performed at the Battelle-Geneva Research Center starting after its invention in 1967. The development work was sponsored over nearly two decades by Daimler-Benz in Stuttgart, Germany, and by Volkswagen AG within the framework of Deutsche Automobilgesellschaft, now a subsidiary of Daimler AG. The batteries showed high specific energy up to 50 W·h/kg (180 kJ/kg), power density up to 1000 W/kg and a reasonable life of 500 charge cycles (at 100% depth of discharge). Patent applications were filed in European countries (priority: Switzerland), United States and Japan and the patents transferred to Daimler-Benz.
Interest grew in the 1970s with the commercialization of the Nickel–hydrogen battery for satellite applications. Hydride technology promised an alternative much less bulky way to store the hydrogen. Research carried out by Philips Laboratories and France's CNRS developed new high-energy hybrid alloys incorporating rare earth metals for the negative electrode. However, these suffered from the instability of the alloys in alkaline electrolyte and consequently insufficient cycle life. In 1987, Willems and Buschow demonstrated a successful battery based on this approach (using a mixture of La0.8Nd0.2Ni2.5Co2.4Si0.1) which kept 84% of its charge capacity after 4000 charge-discharge cycles. More economically viable alloys using mischmetal instead of lanthanum were soon developed and modern NiMH cells are based on this design.
Ovonic Battery Co. in Michigan altered and improved the Ti-Ni alloy structure and composition according to their patent[10] and licensed NiMH batteries to over 50 companies worldwide. Ovonic's NiMH variation consisted of special alloys with disordered alloy structure and specific multicomponent alloy compositions. Unfortunately, linked to their composition, the calendar and cycle life of such alloys always remains very low, and all NiMH batteries manufactured at the present time consist of AB5-type rare earth metal alloys.
Positive electrode development was done by Dr. Masahiko Oshitani from GS Yuasa Company, who was the first to develop high-energy paste electrode technology. The association of this high-energy electrode with high-energy hybrid alloys for the negative electrode led to the new environmentally friendly high-energy NiMH cell.
Currently, more than 2 million hybrid cars worldwide are running with NiMH batteries, e.g., Prius, Lexus (Toyota), Civic, Insight (Honda), Fusion (Ford), and others. Many of these batteries are manufactured by PEVE (Panasonic) and Sanyo.
In the EU and due to the Battery Directive, Nickel–metal hydride batteries have replaced Ni–Cd batteries for portable use by consumers.
Applications
High power Ni-MH Battery of Toyota NHW20 Prius, Japan
Nickel–metal hydride 24V battery pack made by VARTA, Museum Autovision, Altlussheim, Germany
Applications of NiMH electric vehicle batteries includes all-electric plug-in vehicles such as the General Motors EV1, Honda EV Plus, Ford Ranger EV and Vectrix scooter. Hybrid vehicles such as the Toyota Prius, Honda Insight, Ford Escape Hybrid, Chevrolet Malibu Hybrid, and Honda Civic Hybrid also use them. NiMH technology is used extensively in rechargeable batteries for consumer electronics, and it will also be used on the Alstom Citadis low floor tram ordered for Nice, France; as well as the humanoid prototype robot ASIMO designed by Honda. NiMH batteries are also commonly used in remote control cars.
Comparison with other battery types
NiMH cells and chargers are readily available in retail stores in the common sizes AAA and AA. Adapter sleeves are available to use the more common AA size in C and D applications. The sizes C and D cells are somewhat available, but are often just a AA core hidden in an outer shell, with a rating of about 2500 mA·h, much less than ordinary alkaline C and D batteries.[citation needed] Real NiMH C and D batteries are expensive (and the chargers are uncommon); they should be rated at least 5000 mA·h for C and 10,000 mA·h for D sizes.
PP3 (nine volt) NiMH batteries are available; these usually have an output voltage of 8.4 V (1.2 × 7) and a capacity of roughly 200 mA·h. Also available are eight-cell nine volt batteries with a nominal output voltage of 9.6 V (1.2 × 8).
NiMH cells are not expensive, and the voltage and performance is similar to primary alkaline cells in those sizes; they can be substituted for most purposes. Although alkaline cells are rated at 1.5 volts and NiMH cells at 1.2 volts, during discharge the alkaline voltage eventually drops below that of NiMH. This is particularly true for high drain applications, where the voltage of even a fresh alkaline battery can be lower than a NiMH battery while under a load. Furthermore, NiMH batteries offer a flatter discharge curve, particularly at higher current draw.
NiMH cells are often used in digital cameras and other high drain devices, where over the duration of single charge use they outperform primary (such as alkaline) batteries. Applications that require frequent replacement of the battery, such as toys or video game controllers, also benefit from use of rechargeable batteries. With the development of low self-discharge NiMHs (see section above), many occasional-use and very low-power applications are now candidates for NiMH cells.
NiMH cells are particularly advantageous for high current drain applications, due in large part to their low internal resistance. Alkaline batteries, which might have approximately 3000 mA·h capacity at low current demand (200 mA), will have about 700 mA·h capacity with a 1000 mA load.Digital cameras with LCDs and flashlights can draw over 1000 mA, quickly depleting alkaline batteries. NiMH cells can deliver these current levels and maintain their full capacity.
Certain devices that were designed to operate using primary alkaline chemistry (or zinc–carbon/chloride) cells will not function when one uses NiMH cells as substitutes. However, this is rare, as most devices compensate for the voltage drop of an alkaline as it discharges down to about 1 volt. Low internal resistance allows NiMH cells to deliver a near-constant voltage until they are almost completely discharged. This will cause a battery level indicator to overstate the remaining charge if it was designed to read only the voltage curve of alkaline cells. The voltage of alkaline cells decreases steadily during most of the discharge cycle.
Lithium ion batteries have a higher specific energy than nickel–metal hydride batteries,but they are significantly more expensive to produce. In October 2009, ECD Ovonics announced that their next-generation NiMH batteries will provide specific energy and power that are comparable to those of lithium ion batteries at a cost that is significantly lower than the cost of lithium ion batteries

Silver Zinc Batteries |
| Also called Silver Zinc Batteries |
|
Characteristics
Common low capacity primary button cell versions are typically called Silver Oxide batteries. Higher capacity versions available as secondary cells are more often referred to as Silver Zinc batteries. They have an open circuit voltage of 1.6 Volts. Two types of Silver Oxide batteries are available, one type with a sodium hydroxide (NaOH) electrolyte and the other with a potassium hydroxide (KOH) electrolyte.
Because of the high cost of sliver they are available in either very small sizes as button cells where the amount of silver used is small and not a significant contributor to the overall product costs or they are available in very large sizes for critical applications where the superior performance characteristics of the silver oxide chemistry outweigh any cost considerations.
Advantages
High capacity per unit weight.
Long operating life. A tiny button cell will keep a watch running 24 hours per day for 3 to 5 years!!
Low self discharge and hence long shelf life (better than zinc air)
Better low temperature performance than zinc air
Flat discharge characteristics - flatter than the Alkaline Manganese Dioxide battery.
Higher voltage than zinc mercury cells..
Shortcomings
Uses expensive materials.
Lower energy density than zinc air.
Poor low temperature performance.
Limited cycle life.
Suffers from dissolving of the Zinc and the formation of Zinc dendrites which pierce the separator.
Applications
A major contribution to miniature power sources.
As a button cell it is well suited for hearing aids, instruments, photographic applications, electronic watches and other low power devices.
Larger size Silver Zinc batteries are used in submarines, missiles, underwater and aerospace applications.
Silver Zinc secondary cells being promoted as a safer alternative to Lithium cells. Plans to mitigate the higher costs by implementing a recycling programme.
Costs
More expensive than zinc air
Very expensive for high power applications





